Our microscope assembly includes an LED array, foldscope lenses, and a cellphone. The first design for our microscope stand used a LEGO base to hold the LED array in place beneath the foldscope and cellphone arrangement, which were supported by a piece of cardboard. The second design, shown below, was made in Autodesk and 3D printed in the college makerspace.
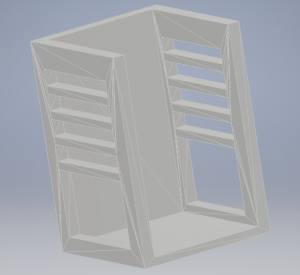
The base of the stand is scaled to snugly hold the LED array in place. The four narrow supports along the sides of the stand are intended to hold the phone, foldscope, and slide assembly in place at four different heights, allowing for very crude z-distance adjustment.
Because the 3D design is uncomplicated, it was decided that future prototypes should be made of laser cut acrylic pieces and fitted together. The 3D printing job was too large to print on a practical timetable, and during early prototyping stages rapid iteration is desirable. The laser cuts take only a few minutes at a time, while the 3D print job took more than 24 hours.
This simple stand design was useful for developing skills in Autodesk and 3D printing, and for holding the microscope setup steady while attempting to take calibration images. However, it does not allow any fineness in z-distance adjustment and no movement at all in the x and y directions. Future designs will include 3 stepper motors arranged so that minute changes can be made in all three directions for best image quality and control.