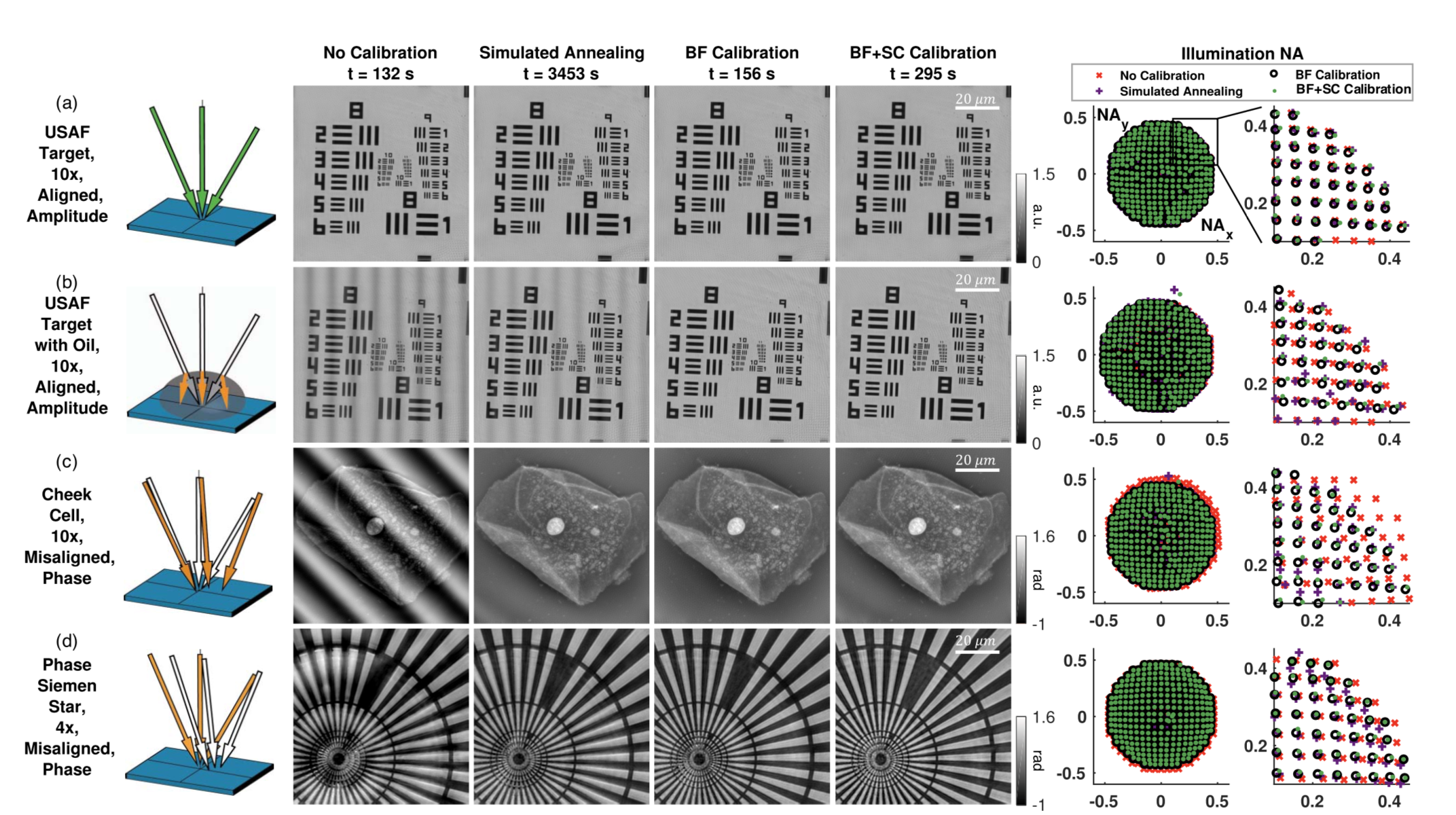
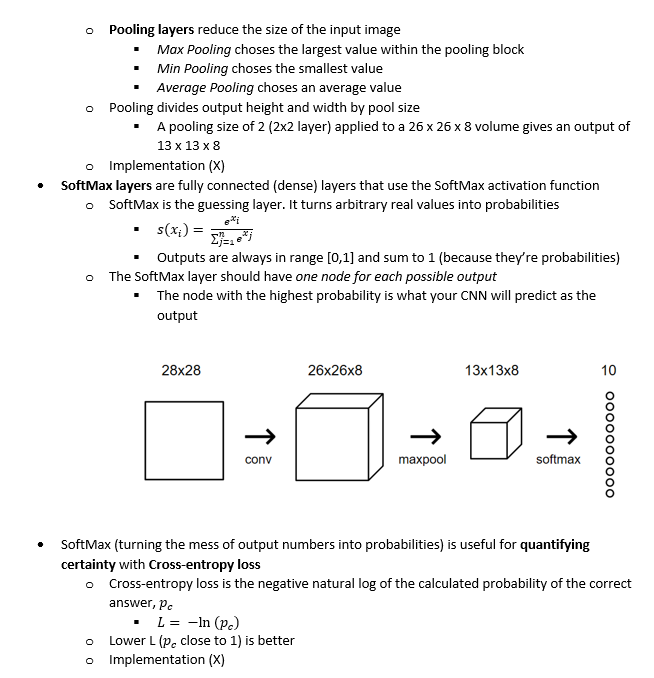
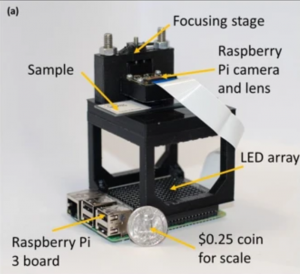
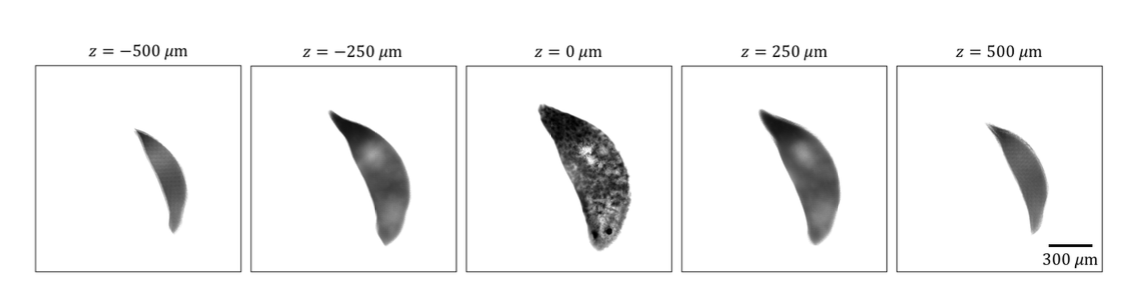
In biological microscopy, a high space-bandwidth product (SBP) is desired, meaning a high resolution and high field of view. Having a high SBP often comes at the cost of temporal resolution, a common problem among medical and biological studies. In addition, high quality imaging generally requires expensive lenses and costly equipment such as spacial light modulators for precise illumination of the sample. In this paper, L. Yeh, S. Chowdhury, and L. Waller propose a microscopy technique that is cheap, efficient, and allows for the multi-modal imaging of both fluorescence and phase.
In conventional microscopy techniques, acquiring a high SPB required the use of mechanical scanning, a time-consuming process in which an expensive automated translation stage scans the sample laterally, taking many images which are later stitched together into one image. Efforts to eliminate the need for mechanical scanning have been developed, such as with Fourier Ptychographic microscopy (FPM). As a faster and cheaper replacement to mechanical scanning, FPM uses an LED array and low numerical aperture (cheaper lens) with a high field of view to take many LR images. A computer algorithm then combines these LR images into one HR image, somewhat speeding up the imaging process while maintaining a high SBP.
This paper discusses a similar technique to FPM, but instead uses structured illumination microscopy (SIM) to capture both incoherent florescence images and coherent phase images. Most conventional types of microscopy are able to only produce either fluorescence images or phase imaging, but not both. This can be disadvantageous, since both intensity and phase images contain different types of useful information about the sample. In this paper, the authors describe a technique of SIM that allows for the acquisition of both types, all while maintaining a high SBP and having a cost-effective setup.
SIM uses the idea of Moiré ‘s Law to aid in super resolution. Moiré‘s Law can be seen on certain images or on television, producing a wavy pattern. This Moiré pattern occurs when an object that is being photographed contains some sort of pattern, like stripes or dots, that exceed the resolution of the sensor. This is the same idea as “beats” in sound, and happens as a result of the interference of waves.

SIM technology takes advantage of the Moiré effect in order to extract higher resolution information from the image. In SIM, a striped or dotted illumination pattern is placed just before the sample. This pattern excites the sample, causing it to fluoresce. The interference of the fluorescence emission and excitation waves creates a Moiré pattern, which can be used and interpreted by a computer algorithm to find more spatial information about the image. This in turn allows for the enhancement of the resolution of the image.
In this paper, Scotch tape is placed just before the sample and mounted on a stage. This Scotch tape contains unknown illumination patterns (a type of SIM known as blind SIM) that can be used to excite the sample and produce a Moiré pattern. A computer algorithm then reconstructs the sample’s super-resolution fluorescent and phase images and corrects any aberrations or out of focus blur. The process is cheap, requiring a low NA and therefor a cheaper lens, yet still manages to have a resolution gain of 4× the original resolution of the objective.
Overall, this particular SIM setup is low-cost, able to capture both incoherent fluorescence images and coherent phase images, and still achieves a high SBP. The authors of this paper claim to be the first to accomplish all three of these at once. Some drawbacks, which could be improved in future work, include the still relatively slow data-acquisition time, which could potentially be sped up via a deep learning framework, and the fact that SIM does not work as well with thick samples.
References:
L. Yeh, S. Chowdhury, and L. Waller. “Computational Structured Illumination for High-Content Fluorescence and Phase Imaging.”Department of Electrical Engineering and Computer Sciences, University of California, Berkeley. Biomedical Optical Express 10. 1978-1998 (2019).
A. Lavdas “Structured Illumination Microscopy–An Introduction.” Bitesizebio.com
N. Mansurov. “What is Moiré?” PhotographyLife.com (2012).
L. Yeh, L. Tian, and L. Waller. “Structured Illumination Microscopy with Unknown Patterns and a Statistcal Prior.”Department of Electrical Engineering and Computer Sciences, University of California, Berkeley. Biomedical Optical Express 8. 695-711 (2017).